Oh, young aspirants! Today, we’re going to tackle two important terms you’ll often hear in your competitive exam preparations: Calcination and Roasting. Don’t worry, even though they sound a bit complex, we’ll break them down into super-easy, bite-sized pieces, just like your favorite snacks!
Think of this as a fun detective mission where we uncover the secrets behind these two processes. By the end of this post, you’ll be a pro at telling them apart!
📚 Related Posts:
◾Explore all the “difference between” Topics
◾Explore all “difference between” Topics on Chemistry
What is Metallurgy?
Metallurgy is the process of extracting pure metal from its ore (a naturally occurring mineral from which metal is extracted).
What is an Ore?
An ore is a rock that contains a metal compound mixed with impurities like sand, clay, etc.
To get pure metal, we first need to remove these impurities. One important step in this process is either Calcination or Roasting.
The Big Reveal: Calcination vs. Roasting!
You might be thinking, “Are they just two fancy words for the same thing?” Nope! While both Calcination and Roasting involve heating materials, they have different goals and are used for different types of substances. Let’s find out how!
What is Calcination? 🔥
Imagine you have a big, heavy rock that contains some useful metal, but it also has things like water or carbon dioxide stuck inside. To get rid of these unwanted guests, we use Calcination!
In simple words, Calcination is the process of heating an ore (that’s the rock with the metal) in the absence of air or with a limited supply of air. Think of it like baking a cake in an oven without letting too much air in.
What happens during Calcination?
- Removal of moisture: Any water molecules trapped in the ore get evaporated and leave.
- Decomposition of carbonates: If the ore contains carbonate compounds (like calcium carbonate, found in limestone), they break down into metal oxides and carbon dioxide gas. For example:
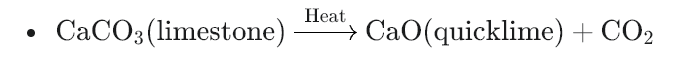
- Decomposition of hydroxides: Similarly, hydroxide compounds break down into metal oxides and water vapor. For example:
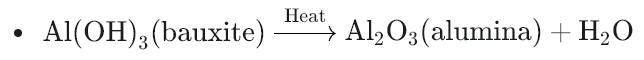
Why do we do Calcination?
- To make the ore porous: Removing water and gases creates tiny holes, making it easier for other chemicals to react with the ore later.
- To remove volatile impurities: These are things that easily turn into gas and escape when heated.
- To convert carbonates/hydroxides into oxides: Metal oxides are often easier to work with for extracting the pure metal.
Think of it this way: Calcination is like cleaning out a cupboard – you’re getting rid of all the unnecessary stuff (like water and carbon dioxide) to make space for what you really want (the metal!).
What is Roasting? 🔥💨
Now, let’s talk about Roasting. Imagine you have an ore that contains sulfur, and you want to get rid of it. Here’s where Roasting comes in handy!
Roasting is the process of heating an ore strongly in the presence of excess air. This is like roasting marshmallows over a campfire – you need plenty of air for them to turn golden brown!
What happens during Roasting?
- Oxidation of sulfides: If the ore contains sulfide compounds (like zinc sulfide or lead sulfide), they react with the oxygen in the air to form metal oxides and sulfur dioxide gas. This sulfur dioxide gas is usually released into the atmosphere or captured for other uses. For example:
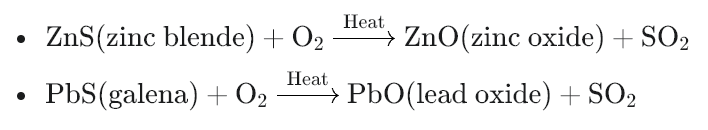
- Removal of impurities: Other impurities that react with oxygen can also be removed as volatile oxides.
Why do we do Roasting?
- To convert sulfide ores into oxides: Metal oxides are generally easier to reduce (meaning, taking away the oxygen) to get the pure metal.
- To remove sulfur impurities: Sulfur is often an unwanted impurity that can make the final metal brittle.
Think of it this way: Roasting is like burning off impurities – you’re using air to help those unwanted elements turn into gases and fly away!
Key Differences Between Calcination and Roasting
| Feature | Calcination | Roasting |
|---|---|---|
| Definition | Heating of ore in the absence or limited supply of air. | Heating of ore in the presence of excess air or oxygen. |
| Purpose | To remove volatile impurities like water, carbon dioxide, etc. | To convert sulphide ores into oxides or sulfates. |
| Type of Ore Used | Generally used for carbonate and hydrated oxide ores. | Mostly used for sulphide ores. |
| Chemical Reaction | Endothermic reaction (requires heat input). | Exothermic reaction (releases heat). |
| Gas Released | Usually releases CO₂ or H₂O vapor. | Often releases SO₂ (sulfur dioxide). |
| Oxygen Supply | Limited or no air/oxygen is supplied during heating. | Heated in the presence of excess air/oxygen. |
| Examples of Ores | ◾ ZnCO₃ → ZnO + CO₂ (Zinc carbonate) ◾ CaCO₃ → CaO + CO₂ (Limestone) | ◾ 2ZnS + 3O₂ → 2ZnO + 2SO₂ ◾ 2PbS + 3O₂ → 2PbO + 2SO₂ |
| Appearance of Ore After Process | Often turns powdery and porous. | May become brittle and colored due to oxidation. |
| By-products | Water vapor, carbon dioxide. | Sulfur dioxide, sometimes sulfur trioxide. |
| Main Objective | To make the ore thermally decomposed and remove moisture/CO₂. | To oxidize the ore and make it suitable for reduction. |
Mnemonic to Remember
🎓 “Calcination Cooks Carbonates, Roasting Rules Sulphides”
This quick line will help you remember which process is used for which type of ore.
Which Exams Ask This?
This topic is frequently asked in:
- SSC CGL, CHSL, GD
- RRB NTPC, Group D
- UPSC Prelims (General Science)
- WBCS, BPSC, UPPSC, and other State PSCs
- NTSE, NDA, CDS
📌 Often asked as MCQs, match-the-following, or conceptual questions.
Practice Questions (Sample MCQs)
Q1: Which of the following processes is carried out in the absence of air?
a) Roasting
b) Calcination
c) Smelting
d) Electrolysis
👉 Answer: b) Calcination
Q2: Roasting is used for which type of ore?
a) Carbonate
b) Hydrated
c) Sulphide
d) Oxide
👉 Answer: c) Sulphide
Q3: Which gas is released during roasting?
a) CO₂
b) SO₂
c) H₂
d) NO₂
👉 Answer: b) SO₂
Quick Recap
🔹 Calcination = Heat without air, removes CO₂
🔹 Roasting = Heat with air, removes S as SO₂
🔹 Both give metal oxides needed for next steps like reduction.
Pro Tip for Exams
➡️ Make a flashcard with the comparison table.
➡️ Revise this topic weekly – it’s a high-scoring question.
➡️ Remember: Carbonate → Calcination, Sulphide → Roasting
Stay Motivated!
Science is fun when you understand it simply. Concepts like these are not only easy to remember but frequent in exams. Keep revising, keep practicing!
You’re just one concept away from success. Keep going! 💪📚
👉 Follow us on GKBooks.in for more simplified science notes, exam boosters, and study hacks!
Explore More:
No post found!