Water, a fundamental resource for life, plays a crucial role in various industrial and domestic processes. However, not all water is created equal, and many regions around the world experience what is known as “temporary hardness.”
In this blog post, we will explore the causes of temporary hardness in water, shedding light on the chemistry behind it and offering insights into potential solutions.
Causes of Temporary Hardness of Water in details
The temporary hardness of water is primarily caused by the presence of dissolved bicarbonate minerals, mainly calcium bicarbonate (Ca(HCO3)2) and magnesium bicarbonate (Mg(HCO3)2).
The temporary hardness can be attributed to the following processes:
Dissolution of Bicarbonate Minerals
- Rainwater is naturally slightly acidic due to the presence of dissolved carbon dioxide, which forms carbonic acid (H2CO3) when it reacts with water.
- This weak carbonic acid reacts with calcium carbonate (CaCO3) and magnesium carbonate (MgCO3) minerals in rocks and soil.
- The reactions result in the formation of soluble calcium bicarbonate and magnesium bicarbonate, which remain in solution.
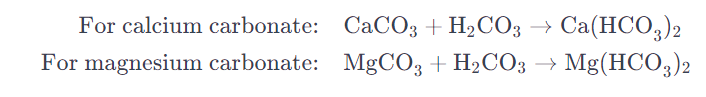
It’s important to note that while temporary hardness can be easily removed by boiling or aeration, permanent hardness is caused by the presence of non-carbonate salts of calcium and magnesium, such as sulfates and chlorides, and is not affected by boiling.
Common Bicarbonate Salts Causing Temporary Hardness
| Bicarbonate Salt | Solubility in Water | Formation Conditions |
|---|---|---|
| Calcium Bicarbonate | Soluble | Presence of CO₂ |
| Magnesium Bicarbonate | Soluble | Presence of CO₂ |
| Sodium Bicarbonate | Soluble | Presence of CO₂ |
Comparative Analysis of Water Softening Methods
| Method | Effectiveness | Cost | Environmental Impact |
|---|---|---|---|
| Boiling | Moderate | Low | Negligible |
| Lime Addition | High | Moderate | Moderate |
| Ion Exchange Processes | Very High | High | Variable |
Impact of Temporary Hardness on Daily Life
Soap Efficiency
- Temporary hardness can affect the efficiency of soap and detergents. Calcium and magnesium ions in hard water form insoluble salts with fatty acids in soap, creating soap scum. As a result, more soap may be required to produce lather and effectively clean, leading to increased soap consumption.
Scaling in Household Appliances
- When hard water is heated, as in water heaters, kettles, and boilers, the dissolved calcium and magnesium ions can precipitate and form solid deposits.
- This buildup is known as scale or limescale, and it can accumulate on heating elements, pipes, and the interior surfaces of appliances.
- Scaling reduces the efficiency of heating elements, making appliances less effective and energy-efficient over time.
Effect on Plumbing Systems
- The accumulation of scale in pipes can lead to reduced water flow and increased pressure in plumbing systems.
- Over time, this can result in clogs and blockages, requiring maintenance and potentially leading to increased repair costs.
Increased Energy Consumption
- Appliances that heat water, such as water heaters and boilers, are particularly affected by temporary hardness. The insulating effect of scale on heating elements can lead to increased energy consumption as more energy is needed to heat water through the insulating layer of scale.
Shortened Appliance Lifespan
- Scaling not only reduces the efficiency of appliances but can also contribute to a shortened lifespan. The accumulation of scale can lead to corrosion, overheating, and mechanical failures in appliances, requiring more frequent replacements.
Environmental Consequences
Energy Consumption and Emissions:
- The increased energy consumption associated with heating hard water contributes to higher greenhouse gas emissions. As more energy is needed to overcome the insulating effect of scale, the environmental impact of energy-intensive appliances is exacerbated.
Waste Generation
- The maintenance and replacement of appliances affected by hard water scaling can result in the generation of electronic waste. This waste may contain hazardous materials, and its improper disposal can contribute to environmental pollution.
Water Treatment Costs
- Municipal water treatment facilities may incur additional costs in treating hard water to make it suitable for distribution. The processes involved in water softening or conditioning may require chemicals and energy, contributing to the environmental footprint of water treatment.
To mitigate these impacts, water softening methods can be employed to reduce the hardness of water, such as ion exchange, chemical softening, or using water softeners. These methods help prevent scaling in appliances, improve soap efficiency, and contribute to more sustainable water usage.
Frequently Asked Questions (FAQs)
Q1: Is temporary hardness harmful to health?
Temporary hardness is not harmful to health; it mainly affects the quality of water for industrial and domestic purposes.
Q2: Can boiling water remove temporary hardness completely?
Boiling water can help reduce temporary hardness by causing the precipitation of calcium carbonate, but it may not eliminate it.
Q3: Are there any natural methods to soften water?
Yes, some natural sources, like peat and zeolites, can aid in softening water. However, their efficiency may vary.
Q4: How can I test the hardness of water at home?
Water hardness test kits are readily available and provide a quick and simple way to measure the hardness of water in your household.
Conclusion
Temporary hardness in water is a prevalent issue that affects various aspects of our daily lives. Understanding the chemistry behind it empowers us to explore effective methods for water softening, ensuring the optimal use of this precious resource. Whether you’re a student, a homeowner, or an industry professional, this knowledge can help you make informed decisions about water treatment and contribute to a more sustainable future.
More Chemistry Topics: