Isobars are elements that share the same mass number but have different atomic numbers, meaning they have distinct chemical properties but identical physical properties. The variation in their chemical properties arises from the different number of electrons in each element. The term “isobars” was introduced by Alfred Walter Stewart in 1918 and comes from the Greek words “Isos,” meaning “equal,” and “baros,” meaning “weight.”
In this article, we’ll explore isobars in more detail, including their properties, applications, and how they compare to isotopes.
What is Isobars in Chemistry?
Isobars are atoms that have the same mass number but different atomic numbers. This means they have different atomic structures, leading to distinct chemical properties.
The atomic number, which indicates the number of protons in an atom, determines its chemical behavior. Therefore, even though isobars have the same mass number—meaning the total number of protons and neutrons in their nuclei is identical—they are different chemical elements with unique chemical properties.
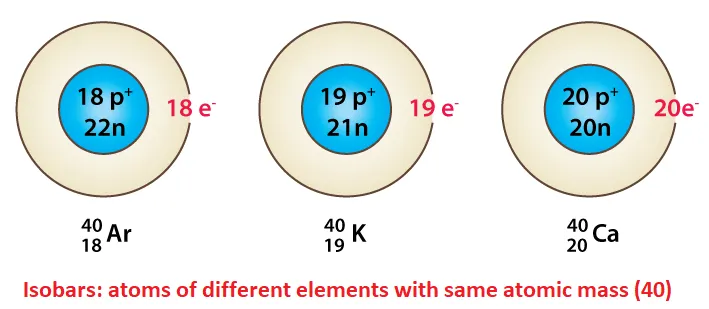
For example, Argon (Z=18), Potassium (Z=19), and Calcium (Z=20) are isobars because they all have a mass number of 40.
Properties of Isobar
Here are some key properties of isobars:
- Same Atomic Mass, Different Atomic Numbers: Isobars are elements that share the same atomic mass but have different atomic numbers, meaning the total number of protons and neutrons in their nuclei is the same, even though the number of protons (and electrons) differs.
- Chemically Distinct: Since isobars have different atomic numbers, they have different electronic configurations and, therefore, different numbers of valence electrons. This leads to distinct chemical properties for each isobar.
- Similar Physical Properties: Isobars have similar physical properties because they have the same mass number, which gives them the same overall mass.
Difference between Isobar and Isotope
Here’s a clear comparison between isobars and isotopes:
| Property | Isobars | Isotopes |
|---|---|---|
| Atomic Number | Isobars have different atomic numbers. | Isotopes have the same atomic number. |
| Mass Number | Isobars share the same mass number. | Isotopes have different mass numbers. |
| Chemical Elements | Isobars represent different chemical elements. | Isotopes are variations of the same chemical element. |
| Electronic Configuration | The electronic configuration of isobars differs. | Isotopes have the same electronic configuration. |
| Number of Electrons | Isobars have different numbers of electrons. | Isotopes have the same number of electrons. |
| Periodic Table Position | Isobars occupy different positions in the periodic table. | Isotopes occupy the same position in the periodic table. |
| Chemical Properties | Isobars have different chemical properties. | Isotopes share the same chemical properties. |
| Physical Properties | Isobars have similar physical properties due to identical mass. | Isotopes have different physical properties. |
| Atomic Structure | Isobars have different atomic structures. | Isotopes have the same atomic structure. |
Uses of Isobars
Here are some uses of isobars:
- Nuclear Physics and Radioactivity: Isobars are commonly studied in nuclear physics, particularly in the context of beta decay. They naturally occur in these processes, making them important for understanding nuclear reactions and transformations.
- Cancer Treatment: Certain isobars, such as those of Cobalt, are used in radiation therapy to treat cancer. Their ability to emit radiation makes them effective in targeting and destroying cancerous cells.
- Age Determination of Planets: Isobars can be used to determine the age of a planet, unlike isotopes. This application is crucial in planetary science and helps researchers understand the history and formation of celestial bodies.
- Goiter Treatment: Iodine isobars are used in the medical treatment of goiter, a condition related to thyroid gland enlargement. The use of these isobars helps in regulating thyroid function.
These applications highlight the significance of isobars in various fields, from medicine to planetary science.
Isobars FAQs
An isobar has the same atomic mass but a different atomic number because the additional neutrons compensate for the difference in the number of protons (nucleons) in the nucleus. This balance ensures that even though the atomic numbers (and therefore the number of protons) differ, the overall mass number remains the same.
Isobars are typically found in different elements because they have the same mass number but different atomic numbers. Since hydrogen only has one proton and no neutrons (in its most common form), it doesn’t have other elements with the same mass number to pair with as an isobar. Therefore, hydrogen cannot be expressed as an isobar.
No, Isobars are atoms from different chemical elements that share the same atomic mass. On the other hand, isotones are atoms from different elements that have the same number of neutrons in their nucleus.
Here are some common examples of isobars:
🔸Argon-40 (1840Ar), Potassium-40 (1940K), and Calcium-40 (2040Ca).
🔸Iron-58 (2658Fe) and Nickel-58 (2858Ni).
🔸Cobalt-64 (2764Co) and Nickel-64 (2864Ni).
These elements have the same mass number but different atomic numbers, meaning they have different numbers of protons and neutrons.
No, isobars do not have the same chemical properties. While they have the same mass number, they are atoms of different elements with different atomic numbers. This means they have different numbers of protons and electrons, which are the primary determinants of an element’s chemical behavior.
For example, Argon-40 (18Ar40), Potassium-40 (19K40), and Calcium-40 (20Ca40) are isobars, but they exhibit very different chemical properties because they belong to different groups in the periodic table and have different electron configurations.


