In Physics, it’s important to understand how materials behave. This behavior depends on the number of tiny particles, like electrons, protons, and neutrons, in their atoms. Elements that have the same number of protons and electrons but a different number of neutrons are called isotopes. Some isotopes occur naturally, while others are made by humans.
This article will explain different isotopes and their properties.
What is an Isotope?
Isotopes are elements that have the same number of protons and electrons but different numbers of neutrons. This means that isotopes have a different number of nucleons (which are protons and neutrons combined).
The properties of elements are determined by the number of tiny particles inside them, called subatomic particles. When the number of these particles changes, the properties of the elements can also change. These changes can happen naturally or by hitting the element with high-speed particles carrying different charges. Here’s an example to help explain:
In the example of hydrogen, it exists in three different forms. Each form has the same number of protons and electrons. The only difference is the number of neutrons. The last two forms are called isotopes of hydrogen and are known as deuterium and tritium.
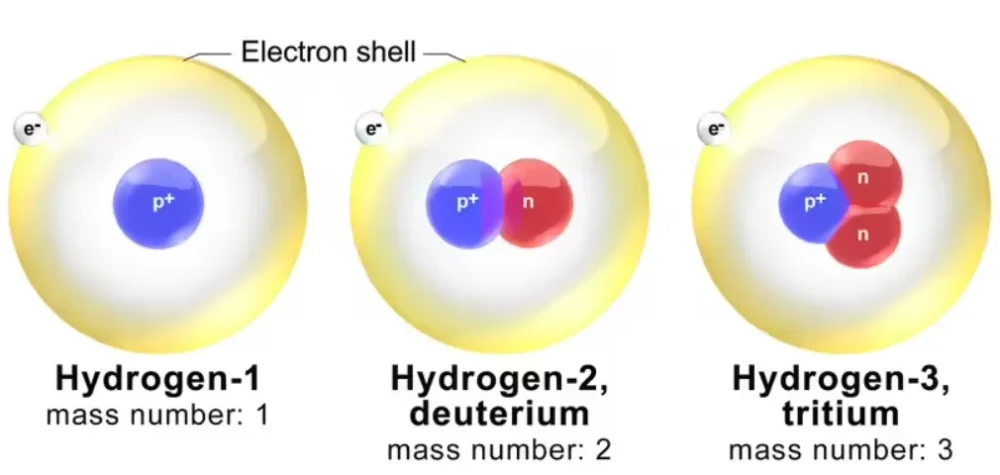
Types of Isotopes
Isotopes can be grouped into different types based on how stable they are:
Stable Isotopes
- As the name suggests, these isotopes remain stable for a long time, and their nuclei do not break down easily.
- When the nucleus of an atom breaks down on its own, it’s called radioactivity. The time it takes for the radioactivity of an element to reduce to half its original amount is called the half-life of that element.
- Stable isotopes have a long half-life.
- In stable isotopes, the number of neutrons and protons is almost equal, usually close to a 1:1 ratio.
- Stable isotopes are used in many applications, like being used as tracers in biological studies.
Primordial Isotopes
- These isotopes have a very long half-life, almost as old as the Earth itself, and some even existed before the Earth was formed.
- Most naturally occurring isotopes are primordial isotopes because their half-lives are nearly as long as the age of the Earth.
Unstable Isotopes
- Unstable isotopes have a short half-life.
- They break down or change often, which makes them radioactive.
- These isotopes change into different, more stable elements over time.
Kinetic Isotope Effect
The isotope effect refers to how the characteristics of an element can change based on the mass of its isotope. When isotopes are used in chemical reactions, they can react at different rates. This difference in reaction rates is known as the kinetic isotope effect.
Isotopes of Elements
Here is a list of different isotopes for some common elements:
Hydrogen
- 1H
- 2H (Deuterium)
- 3H (Tritium)
Carbon
- 12C
- 13C
- 14C
Nitrogen
- 13N
- 15N
Sodium
- 22Na
- 24Na
Potassium
- 40K
- 41K
Uranium
- 232U
- 233U
- 234U
- 235U
- 236U
- 238U
Characteristics of Isotopes
- Isotopes have different mass numbers.
- Their chemical properties are the same because they have the same number of protons.
- They have different physical properties, such as mass, density, melting point, and freezing point.
- Unstable isotopes are radioactive.
- Isotopes are in the same position on the periodic table.
Position of Isotopes in the Modern Periodic Table
In the periodic table, elements are arranged based on their atomic number. Since isotopes of an element have the same atomic number as the stable form of that element, they are placed in the same position on the modern periodic table.
The difference between Isotope and Isobar
| Aspect | Isotopes | Isobars |
|---|---|---|
| Definition | Atoms of the same element with the same number of protons but different numbers of neutrons. | Atoms of different elements with the same mass number but different atomic numbers. |
| Example | Hydrogen isotopes: Protium, Deuterium, Tritium | Iron-58 and Nickel-58 |
| Atomic Number | Same for all isotopes of an element | Different for each isobar |
| Mass Number | Different for each isotope | Same for all isobars |
| Chemical Properties | Identical for all isotopes of an element | Different for each isobar |
| Physical Properties | Different due to varying mass | Similar due to the same mass number |
Application of Isotopes
Isotopes have a wide range of applications across various fields. Here are some key uses:
Medicine
- Diagnostic Imaging: Radioactive isotopes like Technetium-99m are used in imaging techniques such as SPECT scans to diagnose conditions in organs like the heart, brain, and bones.
- Cancer Treatment: Isotopes such as Iodine-131 are used in radiotherapy to treat thyroid cancer by targeting and destroying cancerous cells.
- Sterilization: Cobalt-60 is used to sterilize medical equipment by killing bacteria and other pathogens through gamma radiation.
Industry
- Radiography: Isotopes like Iridium-192 are used in industrial radiography to inspect the integrity of welds and materials by revealing internal flaws.
- Thickness Gauging: Beta-emitting isotopes are used to measure the thickness of materials like paper, plastic, and metal sheets during manufacturing processes.
Agriculture
- Food Preservation: Radioactive isotopes such as Cobalt-60 are used to irradiate food, killing bacteria and extending shelf life without making the food radioactive.
- Pest Control: The sterile insect technique involves releasing sterilized insects, irradiated with isotopes, to control pest populations by preventing reproduction.
Environmental Science
- Tracing and Dating: Isotopes like Carbon-14 are used in radiocarbon dating to determine the age of archaeological and geological samples.
- Water Studies: Isotopes such as Tritium are used to trace water movement and study hydrological processes.
Research
- Biological Studies: Stable isotopes are used to study metabolic processes and nutrient cycles in plants and animals.
- Nuclear Physics: Isotopes are used in experiments to understand nuclear reactions and properties of atomic nuclei.
These applications demonstrate the versatility and importance of isotopes in advancing technology, healthcare, and scientific research.
Hope this article on isotopes helped you understand the topic better! There are many cool topics and their real-life uses to discover. Check out the Gkbooks Facebook page for the latest current affairs, general knowledge, and national and international news. We share infographics that make learning these concepts easy and fun!





