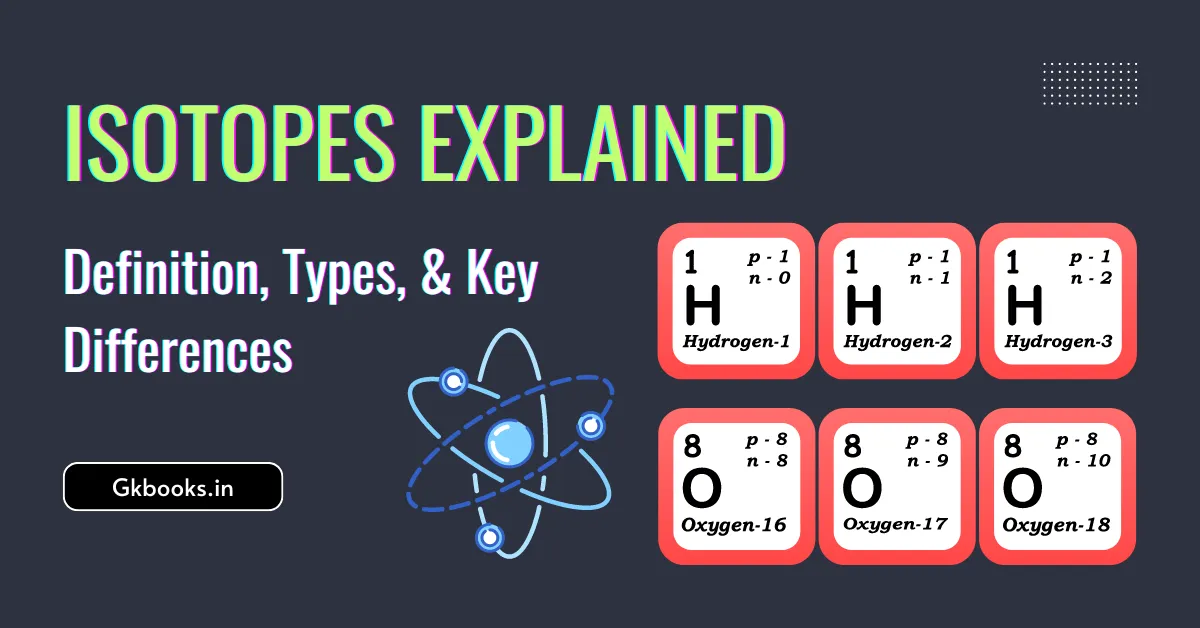
Atomic Number – Know Atomic Mass Number, Isotopes, Isobars & Understand with Examples
The Atomic Number is what makes an atom unique. It’s one of the most basic and important features of an atom. In this article, we will learn about the meaning of atomic number, mass number, and the differences between isotopes and isobars. We’ll also explore the different isotopes of hydrogen and their special traits, along … Read more