The Atomic Number is what makes an atom unique. It’s one of the most basic and important features of an atom. In this article, we will learn about the meaning of atomic number, mass number, and the differences between isotopes and isobars. We’ll also explore the different isotopes of hydrogen and their special traits, along with some solved examples.
Concept of Atomic Number
Henry Moseley discovered the amount of positive charge on an atom’s nucleus in 1913. He used different metals in a discharge tube and directed cathode rays at them. When the rays hit the metals, they emitted unique X-rays. Moseley noticed that the wavelength of these X-rays decreased steadily as he changed the metal in line with its position on the periodic table. He found that the square root of the frequency of these X-rays was directly linked to the number of positive charges in the atom’s nucleus. This led him to conclude that the number of positive charges in the nucleus is the most important characteristic of an atom.
What is an Atomic Number?
The atomic number is a key feature of every atom. It helps identify what element the atom belongs to. Here’s how we can define the atomic number in simple terms:
- The atomic number is the count of positive charges in an atom’s nucleus. These positive charges come from protons, which are tiny particles inside the nucleus.
So, the atomic number of an element is simply the number of protons in the nucleus of its atoms.
Key Points:
- Each proton carries a positive charge.
- In a neutral atom, the number of protons is the same as the number of electrons, which are negatively charged particles surrounding the nucleus.
The atomic number is usually symbolized by the letter Z.
Atomic Number (Z) = Number of Protons = Number of Electrons
Mosley’s Contribution:
Scientist Mosley found a connection between the atomic number and the frequency of X-rays emitted by an element. He described this relationship using the equation:
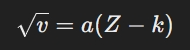
In this equation:
- (v) is the frequency of X-rays.
- (a) and (k) are constants.
- (Z) is the atomic number.
Using this equation, Mosley determined the correct atomic numbers for various elements, fixing some earlier mistakes.
What is Atomic Mass Number?
The mass number of an atom gives us an idea of how heavy the atom is. Here’s a simplified explanation:
Most of an atom’s mass is found in its nucleus, the central part of the atom. The electrons, which move around the nucleus, are so light that they hardly add to the atom’s overall mass.
Inside the nucleus, there are two types of particles:
- Protons (positively charged)
- Neutrons (no charge)
Together, these particles are called nucleons.
Since both protons and neutrons have nearly the same mass (about 1 atomic mass unit each), the total mass of an atom is mainly due to the number of these particles. The mass number is simply the sum of the number of protons and neutrons in the nucleus.
Key Points:
- Mass Number (A) = Number of Protons + Number of Neutrons = Total number of nucleons
- It’s represented by the letter A.
For a neutral atom written as (ZAX):
- Z (atomic number) = Number of protons = Number of electrons
- A – Z = Number of neutrons
This formula helps us understand the basic structure and mass of any atom.
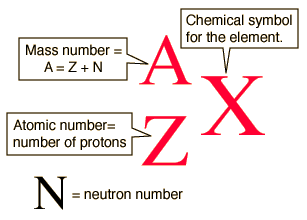
Solved Example: Determining the Number of Electrons, Protons, and Neutrons in an Atom
Let’s find the number of electrons, protons, and neutrons in a chlorine atom represented by 35 17 Cl
General Formula for a Neutral Atom:
For a neutral atom represented as AZX
- Atomic Number (Z): Represents the number of protons.
- Mass Number (A): Sum of the number of protons and neutrons.
The relationships are as follows:
- Number of Electrons = Z
- Number of Protons = Z
- Number of Neutrons = A – Z
Example: Chlorine Atom 3517Cl:
- Atomic Number (Z): 17
- Mass Number (A): 35
Using the formulas:
- Number of Electrons: ( Z = 17 )
- Number of Protons: ( Z = 17 )
- Number of Neutrons: ( A – Z = 35 – 17 = 18 )
Thus, for the chlorine atom 3517Cl:
- Electrons: 17
- Protons: 17
- Neutrons: 18

Difference Between Atomic Number and Atomic Mass Number.
| Atomic Number | Atomic Mass Number |
| The atomic number of an element is the number of positive unit charges in the nucleus of an atom of that element. | The mass number of an atom is the total count of protons and neutrons in its nucleus. |
| The atomic number is important for grouping and identifying an element. | Atomic mass cannot be used to identify the type of element. |
| The atomic number is a number used to arrange elements in the periodic table. | Atomic mass is usually measured in atomic mass units (amu). |
| The atomic number usually represents the number of protons in an element’s nucleus. | Atomic mass is related to the number of protons and neutrons in an element’s nucleus. |
| The letter Z is used to represent the atomic number. | The atomic mass is represented by the letter A. |
| It is the total number of nucleons in the nucleus of an atom, and it represents the average mass of an element. | Atomic mass is also used to differentiate between different isotopes of the same element. Isotopes have the same atomic number but different atomic masses. |
Isotopes and Isobars
Isotopes
Isotopes are different forms of the same element. They have the same number of protons but different numbers of neutrons. This means they have the same atomic number but different mass numbers.
In simpler terms, isotopes are atoms of an element that share the same number of protons but vary in the number of neutrons they contain.
Isotopes of Hydrogen
Hydrogen has three isotopes: protium (also known as proton), deuterium, and tritium.
| Isotope | Symbol | Number of Protons | Number of Neutrons | Number of Electrons | Atomic Mass (u) |
|---|---|---|---|---|---|
| Protium | 1H | 1 | 0 | 1 | 1.008 |
| Deuterium | 2H | 1 | 1 | 1 | 2.014 |
| Tritium | 3H | 1 | 2 | 1 | 3.016 |
Similarly, oxygen has three isotopes, here is a table outlining the isotopes of oxygen:
| Isotope | Symbol | Number of Protons | Number of Neutrons | Number of Electrons | Atomic Mass (u) |
|---|---|---|---|---|---|
| Oxygen-16 | 16O | 8 | 8 | 8 | 15.999 |
| Oxygen-17 | 17O | 8 | 9 | 8 | 16.999 |
| Oxygen-18 | 18O | 8 | 10 | 8 | 17.999 |
Chlorine has two isotopes: 3517Cl and 3717Cl
Isotopes of some elements
| Element | Isotopes |
|---|---|
| Carbon | 12C, 13C, 14C |
| Nitrogen | 14N, 15N |
| Uranium | 233U, 235U, 238U |
| Sulphur | 32S, 33S, 34S, 36S |
✅Uncover Fascinating Facts About Carbon-14, the Radioactive Isotope of Carbon
Characteristics of Isotopes
Here are the key characteristics of isotopes:
- Same Atomic Number: Isotopes of an element have the same atomic number, meaning they have the same number of protons in their nuclei. However, they have different mass numbers because they contain different numbers of neutrons.
- Similar Chemical Properties: Since the chemical properties of an element are determined by the number of protons and electrons, isotopes of the same element show similar chemical behaviors. For example, all isotopes of carbon will produce carbon dioxide when burned.
✅ Discover More In-Depth Information About Isotopes
Isobars
Atoms of different neighboring elements that have the same mass number but different atomic numbers are known as isobars.
- Different Atomic Numbers: Isobars have different atomic numbers, which means they contain different numbers of protons and electrons.
- Same Mass Number: Isobars have the same mass number, so the total number of protons and neutrons is the same for each isobar.
Example: Isobars
For example, the following are isobars:
| Isobar | Symbol | Atomic Number | Number of Protons | Number of Neutrons | Mass Number (u) |
|---|---|---|---|---|---|
| Argon-40 | 40Ar | 18 | 18 | 22 | 40 |
| Potassium-40 | 40K | 19 | 19 | 21 | 40 |
| Calcium-40 | 40Ca | 20 | 20 | 20 | 40 |
Isobars have different chemical properties because they are different elements and occupy different positions in the periodic table. Although they have the same mass number, they are distinct atomic species with varying chemical behaviors.
Difference between Isotopes and Isobars
| Isotopes | Isobars |
|---|---|
| Atoms of the same element. | Atoms of different elements. |
| Have the same atomic number but different atomic mass numbers. | Have the same atomic mass number but different atomic numbers. |
| Have the same number of protons but different numbers of neutrons. | Have different numbers of protons and neutrons. |
| Have the same number of electrons. | Have different numbers of electrons. |
| Occupy the same position in the modern periodic table. | Occupy different positions in the modern periodic table. |
| Exhibit similar chemical properties. | Exhibit different chemical properties. |
| Examples: 12C, 13C, 14C | Examples: 40Ar, 40K, 40Ca |
So, this is all about the Atomic Number. For the latest infographics on General Knowledge and Current Affairs, check out our Facebook Page. Visit now!
Atomic Number FAQs
The number of protons in an atom is equal to the number of electrons. Therefore, the atomic number of an atom, represented by ( Z ), is also equal to the number of electrons present. In summary:
Atomic Number (Z)} = Number of Protons = Number of Electrons
The term atomic number was introduced to describe this fundamental property of an atom. It can be defined as the number of positive unit charges in the nucleus of an atom of a specific element. In simpler terms, the atomic number is the number of protons in the nucleus of an atom.
For sodium, which has 12 neutrons, the complete symbol is 2311Na. Here, 23 is the mass number (protons + neutrons), and 11 is the atomic number (number of protons).
The mass of an atom is largely concentrated in its nucleus. Electrons, which orbit around the nucleus, have such a small mass that they do not significantly contribute to the atom’s overall mass. Therefore, the mass of an atom is essentially the mass of its nucleus.
The nucleus contains protons and neutrons, collectively known as nucleons. Each proton and neutron has a mass close to 1 atomic mass unit (amu). The mass number of an atom is the total count of protons and neutrons in the nucleus, which gives an approximate measure of the atom’s mass in amu.
Mass Number is defined as: The sum of the number of protons and neutrons in the nucleus of an atom.
Isotopes are atoms of the same element that have the same atomic number but different mass numbers. In other words, isotopes are atoms that have the same number of protons but different numbers of neutrons.





