Discover the wonders of calcium carbonate – its formula, properties, uses, and applications.
What is Calcium Carbonate?
Calcium carbonate is a vital inorganic chemical compound represented by the chemical formula CaCO3.
If you’ve ever used chalk in a classroom, you’ve interacted with a form of calcium carbonate. This chemical is abundant in the Earth’s crust and exists in diverse forms like marble and limestone.
In addition to its classroom application, calcium carbonate serves various purposes. It is widely present in nature and plays a significant role in different materials we encounter daily. It’s also found in coral reefs, pearls, and even eggshells! It’s truly a wonder mineral hidden in plain sight.
Calcium Carbonate: A Safe and Odorless Compound
Calcium carbonate is a safe and odorless chemical compound, commonly existing as a white mineral. This compound occurs naturally in abundant sources like chalk, limestone, and marble. It is noteworthy for being non-toxic, making it widely used in various applications without posing harm to living organisms. Let’s delve into its natural occurrences and explore its versatile uses.
Common name of Calcium Carbonate
Calcium carbonate has two main common names, both pretty easy to remember:
- Limestone: This is the most common name for calcium carbonate, especially when it’s found in large rocks like cliffs or mountains. You might have seen pictures of the White Cliffs of Dover – those are made of limestone!
- Chalk: This is a softer type of calcium carbonate often used in classrooms for writing on blackboards. You might also have some in your art supplies if you like pastels or drawing with sticks
The molar mass of Calcium Carbonate
The molar mass of calcium carbonate (CaCO₃) is 100.0869 grams per mole (g/mol). You can calculate it by adding the atomic masses of all its atoms:
- Calcium (Ca): 40.08 g/mol
- Carbon (C): 12.01 g/mol
- Oxygen (O): 16.00 g/mol (multiplied by 3 because there are 3 oxygen atoms in CaCO₃)
So, 40.08 + 12.01 + 3 * 16.00 = 100.0869 g/mol.
Remember, the molar mass tells you the mass of one mole of a substance. So, one mole of calcium carbonate would weigh 100.0869 grams.
Commercial Production of Calcium Carbonate
Commercial Production Grades of Calcium Carbonate
Calcium carbonate is commercially produced in two distinct grades, with their industrial competitiveness primarily hinging on particle size and the characteristics they lend to end products.
Ground Calcium Carbonate (GCC)
- Obtained through extraction and processing of naturally occurring deposits.
- GCC crystals have an irregularly rhombohedral shape and a broader size distribution.
Precipitated Calcium Carbonate (PCC)
- Produced through chemical precipitation via a carbocation process or as a by-product of certain bulk chemical processes.
- PCC crystals exhibit a shape influenced by the specific product, showcasing a more uniform and regular structure with a narrower size distribution.
Distinguishing Factors
- PCC, with smaller particles, boasts higher purity, less abrasiveness, and generally higher brightness compared to GCC.
Calcium Carbonate Structure
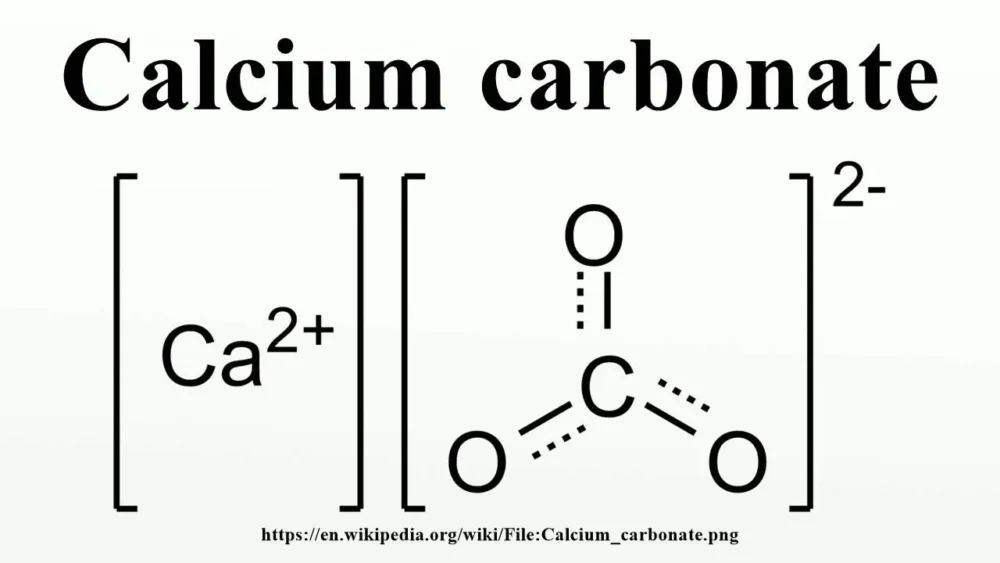
The Formula of Calcium Carbonate
Calcium carbonate, represented by the chemical formula CaCO3, is a chemical compound with diverse applications.
Physical Characteristics
- It presents as a white, insoluble powder-like substance.
- Naturally occurring in minerals, chalk, marble, limestone, calcite, shells, pearls, etc.
Medicinal and Everyday Applications
- Widely employed as an antacid and a calcium supplement in the medical field.
- Used as a filler in cosmetics for its beneficial properties.
Industrial Applications
- Serves as a building material in the form of marble. Acts as a key ingredient for the production of quick lime and cement.
Specialized Uses
- Added to swimming pools as a disinfectant agent and a pH corrector.
Methods of Calcium Carbonate Preparation – CaCO3
Utilizing Carbon Dioxide and Slaked Lime
- Calcium carbonate (CaCO3) is obtained by employing carbon dioxide and slaked lime as raw materials.
- The reaction involves passing carbon dioxide through slaked lime, resulting in the formation of calcite.
- Alternatively, calcite can be obtained by adding sodium carbonate to calcium chloride through the following reactions:
Ca(OH)2 + CO2 → CaCO3 + H2O
CaCl2 + Na2CO3 → CaCO3 + 2NaCl
Large-scale Production through Carbon Dioxide and Calcium Hydroxide
- On an industrial scale, calcium carbonate is prepared by passing carbon dioxide gas through calcium hydroxide (slaked lime).
- However, it is important to note that excess carbon dioxide leads to the formation of soluble calcium hydrogen-carbonate:
Ca(OH)2 + CO2 → CaCO3 + H2O
Understanding the various methods of preparation provides insights into the chemical processes involved in obtaining calcium carbonate. This compound is crucial in different industries, and its preparation methods contribute to its widespread use.
Properties of Calcium Carbonate (CaCO3)
Physical State
- Calcium carbonate exists in the form of a fluffy powder.
Decomposition at High Temperatures
- It undergoes decomposition at elevated temperatures, specifically at 1200K.
- The decomposition results in the release of carbon dioxide.
- Decomposition reaction: CaCO3 → CaO + CO2
Reaction to Dilute Acids
- Calcium carbonate reacts with dilute acids, liberating carbon dioxide as a by-product.
- Example reaction with sulfuric acid: CaCO3 + H2SO4 → CaSO4 + H2O + CO2
- Another example reaction with hydrochloric acid: CaCO3 + 2HCl → CaCl2 + H2O + CO2
Applications of Calcium Carbonate
Pulp and Paper Industry
- Calcium carbonate finds extensive use in the pulp and paper industry.
- It serves as a filter and pigment, enabling the production of a whiter and higher-quality pigment compared to other minerals.
Construction Industry
- In the construction sector, calcium carbonate is utilized as a filler in concrete.
- Its addition enhances the durability and appearance of concrete, contributing to its structural integrity.
- Calcium carbonate is also employed in purifying metals for construction applications.
Agriculture and Fertilizers
- Calcium carbonate plays a vital role in fertilizers, providing essential calcium to plants.
- Additionally, it aids in stabilizing the pH of the soil, promoting optimal conditions for plant growth.
Food Additive and Supplements:
- In the food industry, calcium carbonate serves as an additive for both livestock and human consumption.
- It is used in food products and acts as a supplement in vitamins.
Water and Sewer Treatment
- Water and sewer treatment plants use calcium carbonate in the removal of acidity and impurities.
- Its application contributes to the purification of water for various uses.
Versatile Uses of Calcium Carbonate
Calcium carbonate serves a multitude of purposes across various industries. Here is a list highlighting its diverse applications:
Construction Industry
- Essential as a building material, particularly in the form of marble.
- Acts as a crucial ingredient in cement, contributing to the construction sector.
Medicinal Industries
- Widely employed in medicinal industries for manufacturing antacids.
- Utilized in the production of tablets made from base materials.
Dietary Supplement
- Valued as a calcium supplement, contributing to the nutritional needs of individuals.
Manufacturing Industry
- Integral in the production of paints, enhancing their properties.
- Used in the paper industry for improved quality.
- Plays a role in the plastics industry for certain applications.
Frequently Asked Questions – FAQs
Think of it as your stomach’s superhero! When you suffer from heartburn or indigestion, calcium carbonate, like the one in Tums or Rolaids, neutralizes the excess acid, bringing sweet relief.
Apricots, gooseberries, figs, and raisins raise the calcium flag! Snacking on these delicious friends can help keep your bones and teeth strong.
Absolutely! Limestone is like a giant puzzle built from tiny bits of calcium carbonate, often from the shells and skeletons of sea creatures. It’s used in buildings, sculptures, and even toothpaste!
Think of a science experiment! If you pour acid (like vinegar) on a rock or mineral containing calcium carbonate, it will fizz and bubble. That’s the carbon dioxide escaping, giving you a clear clue!
Chemists use the code CaCO3 to identify this rockstar mineral. Remember, it’s like a team of calcium (Ca), carbon (C), and oxygen (O3) all working together!
More Topics on Chemistry

