An atom is the tiniest part of any element. It contains smaller particles called subatomic particles. The most important of these are electrons, protons, and neutrons.
These particles give the atom many of its key properties. The discovery of electrons, protons, and neutrons came from several interesting experiments.
In this article, we’ll explore how these important subatomic particles were discovered and who made these important discoveries.
Discovery of Electron, Proton and Neutron
For a long time, scientists believed that atoms were the smallest building blocks of matter and could not be divided further.
However, experiments conducted in the late 19th and early 20th centuries showed that atoms are not the final particles.
Scientists discovered that atoms are made up of even smaller particles called subatomic particles.
An atom is made up of three tiny particles: protons, neutrons, and electrons.
Protons and neutrons are found in the nucleus, which is the center of the atom, while electrons orbit around the nucleus.
Protons have a positive charge, and electrons have a negative charge.
The size of the charge on a proton and an electron is the same, but they are opposite in direction.
Neutrons have no charge at all. Protons and electrons attract each other because their charges are opposite.
The discovery of electrons, protons, and neutrons didn’t happen all at once. It was the result of extensive research by many scientists over time. Let’s explore how each of these subatomic particles was discovered.
Discovery of Electrons
Electrons were among the first subatomic particles to be discovered. Here’s a brief overview of how this discovery came about:
In the 1850s, Michael Faraday began investigating electrical discharge in tubes with low air pressure, known as cathode ray discharge tubes. His initial work set the stage for further discoveries.
J.J. Thomson later expanded on Faraday’s research and made the groundbreaking discovery of the electron.
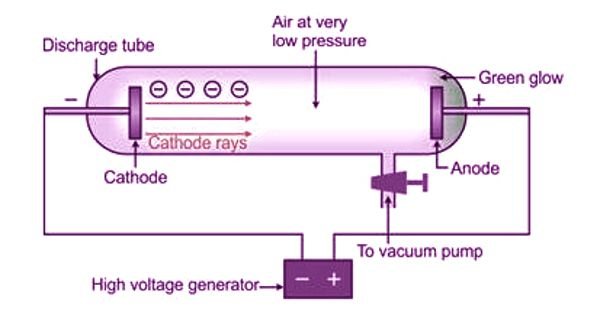
A cathode ray tube is a glass tube with two thin metal pieces called electrodes inside it. The tube is sealed and filled with gas at very low pressure. When a high voltage is applied across the electrodes, a stream of particles moves from the negative electrode (cathode) to the positive electrode (anode).
This stream of particles is known as cathode rays. Thomson’s experiments with cathode rays led to the identification of electrons as tiny, negatively charged particles that are fundamental to the structure of atoms.
Properties of Electron
Electrons are fascinating subatomic particles with several key properties:
- Charge: Electrons carry a negative charge of approximately -1.602 × 10-19 coulombs.
- Mass: The mass of an electron is about 9.109 × 10-31 kilograms, which is roughly 1/2000th the mass of a proton or neutron.
- Location: Electrons are found in the electron cloud surrounding the nucleus of an atom.
- Wave-Particle Duality: Electrons exhibit both particle and wave properties, meaning they can collide with other particles and also show diffraction patterns like light.
- Quantum Mechanics: According to quantum mechanics, the exact position and momentum of an electron cannot be determined simultaneously (Heisenberg’s Uncertainty Principle).
- Role in Atoms: Electrons are responsible for the chemical properties of atoms and participate in forming chemical bonds.
Cathode Ray Tube Experiment
In the 19th century, scientists discovered that when they built a glass tube with wires at both ends and removed most of the air, an electric charge sent through the tube created a fluorescent glow. This setup was often called an “electron gun.”
J.J. Thomson, intrigued by this phenomenon, suspected that the rays produced by the electron gun were connected to the electric charge. To test this, he used a magnetic field in his experiments.
Thomson’s experiment involved placing the glass tube in a partial vacuum, meaning most of the air was removed. He then connected two electrodes at the ends of the tube to a high-voltage power supply. He observed a stream of particles moving from the negative electrode (cathode) to the positive electrode (anode).
The stream of particles was named “cathode rays” because they originated from the cathode. These cathode rays, which are essentially electrons moving from the cathode to the anode, produce an electric current in the tube. This discovery of electrons was a significant breakthrough that transformed our understanding of physics.
Results of this experiment
- Cathode Rays Movement: Cathode rays originate from the cathode and travel towards the anode.
- Straight-Line Travel: Without any electrical or magnetic fields, these rays move in straight lines.
- Behavior in Fields: When subjected to an electrical or magnetic field, cathode rays exhibit behavior typical of negatively charged particles. This indicates that they are made up of negatively charged particles known as electrons.
- Independence from Materials: The properties of cathode rays (electrons) are consistent regardless of the type of electrodes used or the nature of the gas inside the cathode ray tube.
Discovery of Protons
1815: English chemist William Prout proposed that all atoms are made up of fundamental hydrogen atoms.
1886: German physicist Eugen Goldstein discovered canal rays and found that the charge-to-mass ratio of hydrogen ions was the highest among all gases.
1917: Ernest Rutherford experimented using a modified cathode ray tube.
- Rutherford found that when a beam of alpha particles was directed at air, scintillation detectors detected hydrogen nuclei.
- He further investigated and discovered that nitrogen atoms in the air produced hydrogen nuclei.
- Rutherford then fired alpha particle beams into pure nitrogen gas and observed an increase in hydrogen nuclei.
Conclusion: It was concluded that hydrogen nuclei came from nitrogen atoms, suggesting that all atoms contain hydrogen as a fundamental particle. The hydrogen nucleus was later named the “proton” and recognized as a key component of the atomic nucleus.
Properties of Proton
Here are some key properties of protons:
- Charge: Protons have a positive electric charge of +1 elementary charge (e), which is approximately (1.602 \times 10^{-19}) coulombs.
- Mass: The mass of a proton is about (1.672 \times 10^{-27}) kilograms, or approximately 1 atomic mass unit (amu).
- Location: Protons are found in the nucleus of an atom. They, along with neutrons, make up the nucleus, which is the dense central part of an atom.
- Stability: Protons are stable particles and do not decay under normal conditions. Some theories predict that protons may decay over extremely long timescales, but this has not been observed.
- Composition: Protons are made up of three quarks (two up quarks and one down quark) held together by the strong nuclear force, mediated by gluons.
- Role in Elements: The number of protons in the nucleus of an atom determines the element’s identity and its atomic number. For example, hydrogen has one proton, helium has two, and so on.
- Interactions: Protons interact with other particles through gravitational, electromagnetic, weak, and strong forces. The strong force is what binds protons and neutrons together in the nucleus.
Rutherford Gold Foil Experiment
In 1911, Ernest Rutherford, along with Hans Geiger and Ernest Marsden, conducted a series of groundbreaking experiments that transformed the understanding of the atom. They directed a beam of fast-moving alpha particles at an extremely thin sheet of gold foil. Alpha particles are positively charged and have a mass similar to that of a hydrogen atom, making them a type of naturally occurring radioactive particle.
At the time, scientists expected that the alpha particles would pass through the gold foil with minimal deflection, based on the prevailing atomic model, which suggested that an atom’s mass and charge were evenly distributed. However, to their surprise, while most of the alpha particles did pass through, a small fraction (about 1 in 8,000) bounced off at sharp angles, with some even reflecting straight back.
To explain these unexpected results, Rutherford proposed a new atomic model. He concluded that the majority of the atom is empty space, allowing most alpha particles to pass through. The particles that were deflected, however, must have encountered a powerful force within the atom. Rutherford deduced that this force came from a dense, positively charged center, which he called the nucleus. This tiny nucleus contains almost all of the atom’s mass and consists of protons and neutrons.
Rutherford’s findings led to the development of the nuclear model of the atom, with the nucleus at its core, fundamentally altering the understanding of atomic structure.

Discovery of Neutrons
When alpha particles emitted by heavy elements such as polonium were directed at lighter elements like lithium, beryllium, and boron, a penetrating form of radiation was observed. This radiation was unaffected by electric fields, leading scientists to initially believe it was gamma radiation due to its neutral charge.
The radiation carried high energy, approximately 5 MeV, prompting Italian physicist Ettore Majorana to propose the existence of a neutral particle. Similarly, after his alpha scattering experiment, Ernest Rutherford also suggested the possibility of a neutral particle within the atom.
British physicist Sir James Chadwick further investigated this radiation. He discovered that the electrically neutral particles emitted had a mass slightly greater than that of protons. Chadwick named these particles neutrons, marking a significant advancement in atomic theory. This discovery explained the missing mass in the atomic nucleus and contributed to the development of modern nuclear physics.
Properties of Neutrons
- Charge: Neutrons are electrically neutral, meaning they have no net electric charge.
- Mass: The mass of a neutron is approximately (1.675 \times 10^{-27}) kilograms, which is slightly greater than that of a proton.
- Location: Neutrons are found in the nucleus of an atom, along with protons. They help to bind the nucleus together through the strong nuclear force.
- Stability: Free neutrons (those not bound within a nucleus) are unstable and undergo beta decay with a half-life of about 611 seconds. During this decay, a neutron transforms into a proton, an electron, and an antineutrino.
- Composition: Neutrons are composed of three quarks (one up quark and two down quarks) held together by the strong nuclear force, mediated by gluons.
- Role in Elements: The number of neutrons in an atom’s nucleus can vary, leading to different isotopes of an element. For example, carbon-12 and carbon-14 are isotopes of carbon with 6 and 8 neutrons, respectively.
- Interactions: Neutrons interact with other particles through gravitational, weak, and strong forces. They do not interact via the electromagnetic force due to their lack of charge.
If you have any more questions or need further details, feel free to ask!

Non-Fundamental Particles
Particles other than electrons, protons, and neutrons are classified as non-fundamental particles. Here’s a brief overview of some key non-fundamental particles:
- Positron: The positron is the antiparticle of the electron. It carries a positive charge but has the same mass as an electron. It is represented as e⁺.
- Antiproton: This is the antiparticle of the proton. It has a negative charge and the same mass as a proton. It is represented by ‘p⁻’.
- Neutrino and Antineutrino: These particles were proposed during the emission of beta (β) particles from radioactive nuclei. They have no mass or charge, but they carry energy and momentum.
- Pi-mesons (π-mesons): Yukawa predicted the existence of these particles. Nuclear forces are believed to result from the exchange of pi-mesons between nucleons (protons and neutrons). There are three types: positive, negative, and neutral pi-mesons.
- Quarks and Bosons: Quarks are the fundamental building blocks that form heavy subatomic particles like protons and neutrons. Bosons, on the other hand, are particles associated with forces. Bosons can include mesons and photons, and they are characterized by whole number spin values.
In summary: Bosons = Mesons + Photons
These particles help explain complex interactions within atoms and contribute to our understanding of the subatomic world.
So, this concludes the discovery of the Electron, Proton, and Neutron. For more updates on General Knowledge, Science, and Current Affairs, make sure to follow our official Facebook page!
Discovery of Electron, Proton, and Neutron FAQs
The discovery of protons resulted from experiments involving electrical discharge in a modified cathode ray tube.
During these experiments, positively charged particles were observed, leading to the identification of protons as fundamental components of the atom.
Protons carry a positive charge and are located in the nucleus of an atom, playing a crucial role in determining the atomic number and the identity of the element.
Sir James Chadwick is credited with the discovery of the neutron in 1932.
Ernest Rutherford is credited with the discovery of the proton in 1917 through his famous gold foil experiment and subsequent studies on atomic structure.
He identified the hydrogen nucleus, which he later named the proton, as a fundamental particle in atoms.
Because of his groundbreaking work in uncovering the proton’s role in the atomic nucleus, Rutherford is often referred to as the father of the proton.
The term “electron” was coined by the Irish physicist George Johnstone Stoney in 1891.
He introduced the word to describe the fundamental unit of electric charge.
Although the particle itself had not yet been discovered, Stoney’s theoretical work laid the groundwork for its identification.
Later, in 1897, J.J. Thomson experimentally discovered the electron, confirming Stoney’s concept of this subatomic particle.
In 1909, Ernest Rutherford conducted the famous Gold Foil Experiment, which ultimately led to the discovery of the nucleus.
Through this experiment, Rutherford and his team observed that while most alpha particles passed straight through the gold foil, a few were deflected at large angles.
This surprising result led Rutherford to propose that the atom’s positive charge and most of its mass were concentrated in a small, dense region at the center, which he called the nucleus.





