Types of Polymers play an essential role in modern life, shaping our daily routines and enhancing convenience. From everyday items like brushes, plastic cups, and bags to more specialized products such as buckets, toys, synthetic fabrics, tires, and electrical insulation materials, polymers are ubiquitous. Even the proteins within our bodies are polymers, highlighting their fundamental presence. This chapter will delve into the diverse array of polymers, examining their significance and various applications in our lives.
What are Polymers?
- A Polymer is a combination of many repeating subunits.
- The polymers are giant molecules with high molecular masses (103 -107u).
- These repeating subunits are called ‘monomers’.
- The word ‘polymer’ is derived from two Greek words, Poly – means ‘many’ and ‘Mer’ – means ‘unit or part’.
- For example, the monomer ethylene gets linked with many other ethylene molecules to form polyethylene or many vinyl chloride molecules combine to form polyvinyl chloride.
- Polymers can be natural or synthetic. Natural polymers include proteins, cellulose, and rubber.
- Synthetic polymers are man-made and include plastics like polyethylene, polypropylene, and polystyrene.
- Polymers have diverse properties, including flexibility, durability, and electrical conductivity.
- They are used in various industries, such as packaging, construction, textiles, and electronics.
- Polymers play a crucial role in everyday life, from household items to advanced technologies.
Common classifications of polymers
Here are some common classifications of polymers based on special features such as source and structure:
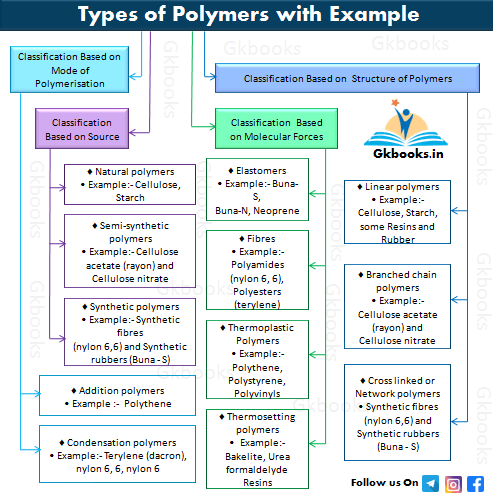
1. Classification Based on Source.
2. Classification Based on Structure of Polymers
3. Classification Based on Mode of Polymerisation
4. Classification Based on Molecular Forces
Classification of Polymers Based on Source
There are three subcategories-
- 1. Natural polymers
- 2. Semi-synthetic polymers
- 3. Synthetic polymers
Natural Polymers
- Natural polymers are the most common type of polymers.
- These polymers are found in both humans and animals.
- Examples include resins and rubber, which are derived from plants.
- Starch and cellulose are other examples of natural polymers found in plants.
- Proteins, which are natural polymers, are found in animals and are essential for various bodily functions.
Semi-synthetic Polymers
- Semi-synthetic polymers are produced artificially in laboratories by modifying natural polymers.
- These modifications enhance the properties of the natural polymers for specific uses.
- Examples of semi-synthetic polymers include cellulose acetate (rayon) and cellulose nitrate, both of which are derivatives of cellulose.
Synthetic Polymers
- Synthetic polymers are artificially produced by humans.
- These polymers are manufactured on a large scale by industries to meet high demand.
- Examples of synthetic polymers include plastics like polyethylene (polythene), nylon 6,6, and synthetic rubbers such as Buna-S.
Classification of Polymers based on the Structure of Polymers
Polymers based on Structure are subdivided into three categories:
- Linear polymers
- Branched-chain polymers
- Cross-linked or Network polymers
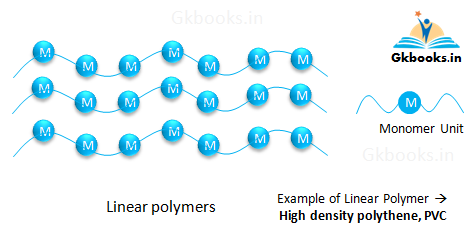
Linear Polymers
- Linear polymers are formed when monomers are united together to create a long, straight chain.
- These polymers have high melting and boiling points, high density, and high tensile strength due to their well-packed structure.
- Examples of linear polymers include high-density polyethylene (HDPE), polyvinyl chloride (PVC), and nylon.
- Linear polymers are commonly used to make bottles, buckets, and PVC pipes because of their strength and durability.
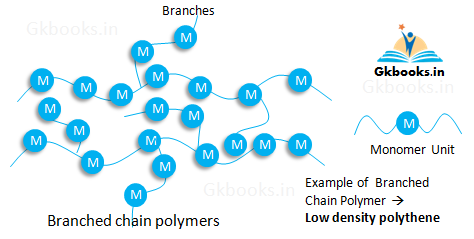
Branched-chain polymers
- Branched-chain polymers consist of a linear chain with branches extending from the main chain.
- These polymers have a low density and low melting points.
- The presence of branches prevents them from packing closely together.
- As a result, they have low melting and boiling points, low density, and low tensile strength.
- An example of a branched-chain polymer is low-density polyethylene (LDPE).
- LDPE is commonly used to manufacture plastic bags, dispensing bottles, tubing, and wash bottles due to its flexibility and durability.
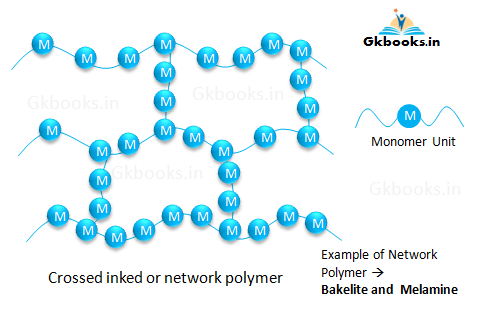
Cross-linked or Network polymers
- Cross-linked or network polymers are formed by bi-functional and tri-functional monomers.
- These monomers are linked together by strong covalent bonds.
- The monomers create a three-dimensional network, resulting in a very stable and rigid structure.
- Examples of cross-linked polymers include Bakelite and Melamine.
- Bakelite is widely used in the manufacturing of electrical insulating materials due to its excellent insulating properties.
- Melamine is primarily used in the manufacturing of floor tiles, kitchenware, and fabrics because of its durability and heat resistance.
Classification based on the Mode of Polymerisation
Based on the mode of polymerization, polymers can also be classified into two subgroups:
- Addition polymers
- Condensation polymers
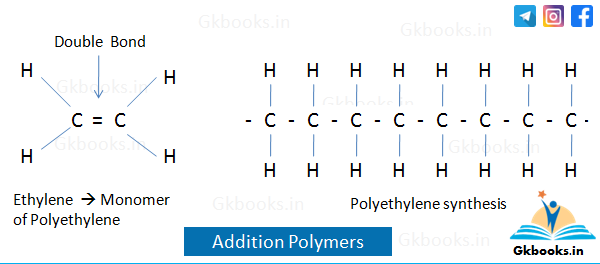
Addition Polymers
- Addition polymers are formed by the repeated addition of monomeric units, which are molecules of a single type of monomer.
- The addition polymerization reaction does not produce any by-products, such as water.
- The monomeric units used in this process are unsaturated and possess double bonds.
- Addition polymers are non-biodegradable and resistant to acids.
- Examples of addition polymers include Polythene (polyethylene) and Polypropene (polypropylene).
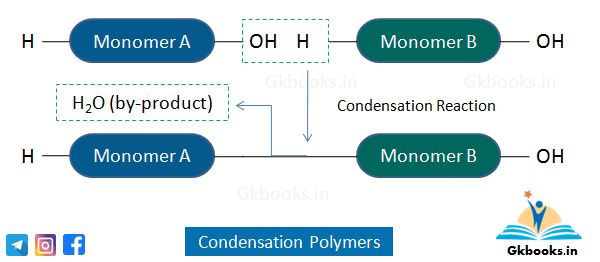
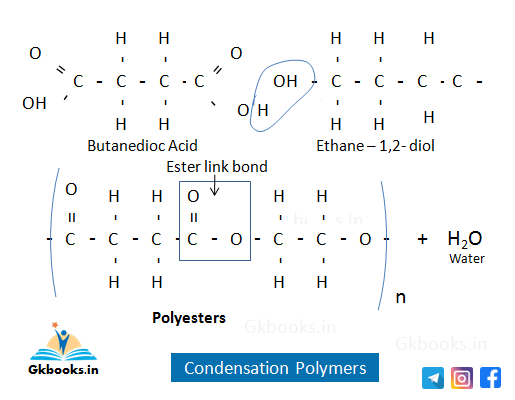
Condensation Polymers
- Condensation polymers are formed by the repeated addition of two different monomeric units (molecules of two different monomers).
- The condensation reaction produces by-products, usually water.
- The monomeric units contain reactive functional groups that react with each other, such as carboxylic acid and alcohol.
- These polymers are biodegradable and can be hydrolyzed by acids.
- Examples of condensation polymers include Nylon 6, Terylene (Dacron), and Nylon 6,6.
Classification based on Molecular Force
The mechanical properties of polymers depend on the intermolecular forces within the polymer. Some of these mechanical properties include elasticity, toughness, and tensile strength. The intermolecular forces governing these mechanical properties include:
- Van der Waals forces
- Presence of hydrogen bonds
Based on the intermolecular forces present in the polymer, polymers are divided into four subclasses:
- Elastomers
- Fibers
- Thermoplastic polymers
- Thermosetting polymers
Elastomers or Rubber
- Elastomers, also known as rubber, have the weakest intermolecular forces of attraction, specifically Van der Waals forces, between their molecules.
- Because of these weak forces, elastomers can be easily stretched when a force is applied and will return to their original shape once the force is released.
- Examples of elastomers include Buna-S, Buna-N, and Neoprene.
Fibers
- Fibers are polymers characterized by strong molecular forces such as hydrogen bonding.
- They possess high tensile strength, meaning they can withstand a lot of pulling force without breaking.
- Fibers also have a high modulus, indicating they are stiff and resistant to deformation.
- Examples of fiber polymers include polyamides like Nylon 6,6 and Polyesters like Terylene.
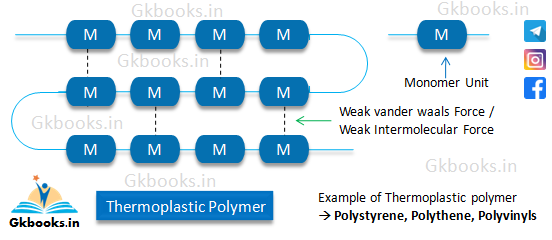
Thermoplastic Polymers
- Thermoplastic polymers are composed of long-chain linear or slightly branched molecules.
- They exhibit the characteristic of becoming soft when heated and hard when cooled, making them easily moldable.
- The intermolecular forces of attraction in thermoplastic polymers are intermediate between elastomers and fibers.
- Weak Vander Waals forces are responsible for the weak intermolecular forces of attraction in thermoplastic polymers.
- The formation of thermoplastic polymers involves addition polymerization reactions.
- Thermoplastic polymers typically have low molecular weights.
- They are soluble in organic solvents, allowing for easy processing and recycling.
- Examples of thermoplastic polymers include polystyrene, polyethylene, and polyvinyls.
Thermosetting Polymers
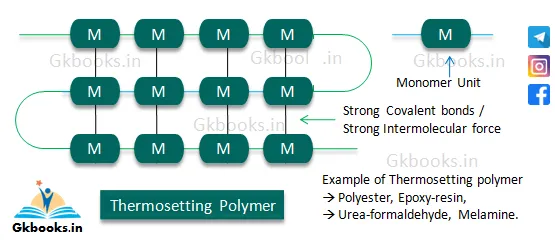
- Thermosetting polymers are heavily branched, cross-linked polymers, also known as thermosets.
- They are sometimes called rigid-temperature polymers due to their inability to soften upon heating.
- The formation of thermosetting polymers involves a condensation polymerization reaction.
- Once formed, thermosetting polymers cannot be softened by heating, as they are held together by strong covalent bonds.
- These polymers are hard, strong, more brittle, and cannot be reused once molded.
- The strong intermolecular force of attraction in thermosetting polymers is due to the presence of strong covalent bonds.
- They have high molecular weights and are insoluble in organic solvents.
- Examples of thermosetting polymers include polyester, epoxy resin, urea-formaldehyde, and melamine.
Classification of Polymers Infographics
List of Polymers, Monomers, and Uses
| Polymers | Monomers | Uses |
|---|---|---|
| Polypropylene | • Propane | • Manufacturing of Ropes, Toys, Pipes, Fibres |
| Polystyrene | • Styrene | • Insulator • Wrapping material • Manufacture of Toys • Manufacture of Radio and Television cabinets |
| Polyesters / Dacron or terylene | • Ethylene glycol • Terephthalic acid | • Used in blending with cotton and wool fibres • Glass reinforcing materials in safety helmets |
| Polyvinyl chloride (PVC) | • Vinyl chloride | • Manufacture of rain coats, hand bags, vinyl flooring, water pipes |
| Glyptal. | • Ethylene glycol • Phthalic acid | • Manufacture of Paints and Lacquers |
| Urea-formaldehyde Resin | • Urea • Formaldehyde | • For making Unbreakable cups and Laminated Sheets. |
| Bakelite | • Phenol • Formaldehyde | • Making combs • Electrical switches • Handles of Utensils • Computer discs |
| Poly β-hydroxybutyrate – co-β-hydroxy valerate (PHBV) | • 3-hydroxybutanoic acid • 3 – hydroxypentanoic acid | • Used in the packaging of orthopedic devices |
| Buna – N | •1, 3 – butadiene • Acrylonitrile | • Used in making oil seals, Tank lining |
| Neoprene | • Chloroprene. | • Used for manufacturing Conveyor belts Gaskets and Hoses. |
| Melamine | • Melamine • Formaldehyde | • Manufacturing of unbreakable crockery |
| Polytetrafluoroethene (Teflon) | • Tetrafluoroethene | • Used in making oil seals and gaskets , non–stick surface coated utensils |
| Polyacrylonitrile (Orlon/Acrilan) | • Acrylonitrile | • Making commercial fibres as orlon or acrilan |
| Nylon 6 | • Caprolactum | • Manufacture of tyre cords, fabrics, and ropes |
| Nylon 6,6 | • Hexamethylenediamine • Adipic acid | • Used in making sheets, bristles for brushes and in the textile industry |
Types of Polymers: Previous Year Questions
Q1. PVC is obtained by the polymerization of? [SSC Section Officer (Audit) 2005]
Answer: Vinyl chloride
Q2. Which polymerization is used in the polyethene manufacturing industry? [SSC Section Officer (Commercial Audit) 2005]
Answer: Ethylene
Q3. A polymeric substance used to make parachutes is? [SSC Tax Assistant (Income Tax & Central Excise) 2005]
Answer: Terylene
Q4. The Polythene is a polymer of? [SSC Section Officer (Audit) 2006]
Answer: Ethylene
Q5. The polymer used in making plastic crockery is? [SSC CGL Prelim 2007]
Answer: Melamine
Q6. What is the name of the plastic polymer from which combs, toys, bowls, etc., can be made? [SSC Combined Matric Level (PRE) 2002]
Answer: Polystyrene
Q7. Natural rubber is the polymer of [SSC MTS 2011]
Answer: Isoprene
Q8. Bakelite is a copolymer of Phenol and? [SSC Stenographer Grade ‘C’ & ‘D’ 2011]
Answer: Formaldehyde
Frequently Asked Questions (FAQ)
Answer: Nylon 66
Answer: Dialkyl dichloro silane
Answer: Isoprene
Answer: Polymer
Answer: Terephthalic acid
Answer: Polyamide polymer
Primary Source of this Article -NCERT
To download all the NCERT books – click Here

